Selected Publication Highlights (full list available here)
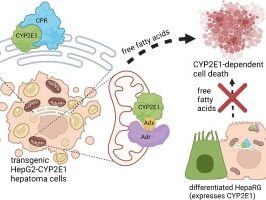
Subcellular expression of CYP2E1 in HepG2 cells impacts response to free fatty acids
Current Research in Toxicology, 2024
Cytochrome P450 2E1 (CYP2E1) is known for its role in metabolizing drugs and pollutants, but it also acts on endogenous fatty acids and ketones. In this study, the Hartman Lab created HepG2 cell lines expressing CYP2E1 targeted to the endoplasmic reticulum, mitochondria, or both to examine how subcellular localization influences responses to the fatty acids palmitate and oleate. Cells expressing CYP2E1 in both organelles showed heightened lipotoxicity and inhibited respiration after palmitate exposure, while ER-targeted CYP2E1 prevented the oleic acid–induced increase in respiration. Interestingly, differentiated HepaRG cells, which express abundant CYP2E1, were resistant to palmitate toxicity. Analysis of patient data revealed lower CYP2E1 expression in liver tumors, with higher expression correlating with better survival outcomes. Together, these findings highlight distinct roles for mitochondrial and ER CYP2E1 in lipid metabolism and suggest a potential protective role for CYP2E1 in liver cancer.
Ethanol metabolism by CYP2E1 alters C. elegans behavior and mitochondria
Biochemical and Biophysical Research Communications, 2024
Cytochrome P450 2E1 (CYP2E1) is best known for breaking down alcohol in the liver, but it is also found in the brain where it may influence alcohol’s neurological effects. In this study, the Hartman Lab created C. elegans and rat PC-12 cell models expressing human CYP2E1 targeted either to mitochondria or the endoplasmic reticulum to understand how its subcellular location shapes responses to ethanol. Worms expressing mitochondrial CYP2E1 were more sensitive to ethanol-induced mitochondrial inhibition, while worms expressing endoplasmic reticulum CYP2E1 recovered faster from ethanol-induced movement loss. Similarly, PC-12 cells expressing mitochondrial CYP2E1 were more vulnerable to ethanol-induced cell death. These findings reveal that the cellular localization of CYP2E1 can determine how alcohol affects both neuronal function and mitochondrial health
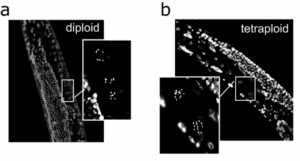
Polyploid worms reveal how extra genomes affect stress resistance
Scientific Reports, 2022
Some cells naturally carry more than two copies of their genome, a state known as polyploidy. Using a new method to generate tetraploid (four-copy) C. elegans, the Hartman Lab investigated how having extra genomes alters physiology and response to stress. Tetraploid worms were larger but had shorter lifespans, smaller brood sizes, and more germline apoptosis and aneuploid embryos than diploids. Surprisingly, they showed only modest protection from the DNA-damaging chemotherapeutics cisplatin and doxorubicin and were equally or more sensitive to reproductive toxicity. Transcriptomic analysis revealed broad suppression of cell-cycle and DNA-repair pathways. Together, the work provides the first integrated view of how whole-animal polyploidy affects development, reproduction, and stress resilience in a living organism.
Worms that exercise have better mitochondria
Scientific Reports, 2018
An average lab worm spends its (on average, 17 days) life endlessly gobbling E. coli. It spends 2.5 days developing to adulthood, about 5 days having offspring, and then lives out its remaining 10 days in a steep decline in overall physiology. We have shown in this paper that, like humans, a worm can age more gracefully if it is more active. If that worm undergoes repeated swim sessions throughout its reproductive adulthood (twice daily exercise for 6 days after reaching adulthood), its mitochondria are healthier as it ages. We found the mitochondria maintain a more normal number and size, and function better. Finally, we found the animals live a little bit longer and are more resistant to lethal doses of the drinking water contaminant arsenic and the pesticide rotenone.
The neurotrophic factor MANF is involved in unfolded protein response and innate immunity in worms
European Journal of Cell Biology, 2019
The mysterious MANF is a neurotrophic factor, which is a small secreted protein that is involved somehow in the growth, survival, or differentiation of neurons. It is the only neurotrophic factor conserved from humans to worms, and it was only recently discovered in worms. We reported in this paper that worms lacking MANF expression had altered unfolded protein response. They were protected from larval growth arrest during exposure to tunicamycin, a chemical inhibitor of protein glycosylation that causes many proteins to be misfolded. Based on a microarray experiment to look at gene expression, we found altered innate immunity pathways in the MANF-deficient animals. Accordingly, we also found they were also protected from growth delay from exposure to a pathogenic bacteria, Pseudomonas aeruginosa.
Genetically diverse mice reveal differences in mitochondrial CYP2E1 activity correlated with butadiene-induced mitochondrial dysfunction
Toxicology, 2017
The Collaborative Cross is a genetically diverse panel of mice used for population genetics studies. We took advantage of the genetic variation in this model and exposed mice from 60 strains to either clean air or a high dose of 1,3-butadiene, a volatile chemical in diesel exhaust and cigarette smoke that is a known carcinogen. We found that these mice exhibited variation in liver CYP2E1 activity, both in the endoplasmic reticulum where it is typically measured and in the mitochondrial fraction. We also found that after exposure, the mice livers had mitochondrial dysfunction. Interestingly, we saw that the severity of mitochondrial dysfunction was correlated with mitochondrial, but not endoplasmic reticulum, CYP2E1 activity. This is the first in vivo evidence for toxicological consequences of mitochondrial CYP2E1 localization.